Research helps us better understand mood disorders, develop new treatments, and learn which approaches work best for different people. At DBSA, we support studies that align with our mission to improve the lives of those living with mood disorders. Participation isn’t for everyone and some studies involve risks, so this page is here to help you learn about research types and make an informed decision.
The Role and Value of Participating in Clinical Research

Two main types of studies
Researchers use different kinds of studies to better understand how conditions affect people and how treatments can improve daily life. These two types of studies work together to guide safer, more effective care.
Observational Studies
- Collect information about people in everyday life
- Do not test a medication or procedure
- Help identify questions to test in future trials
Clinical Trials
- Test medications, procedures, or behavior changes
- Determine whether a treatment is safe and effective
- Compare new options to the current standard care
How Clinical Research Helps People
Clinical research connects real people’s experiences to medical progress.
- Finds better ways to diagnose and treat diseases
- Tests how safe medications are and who they work best for
- Identifies ways to prevent illness in people at higher risk
- Improves the quality of life for people with chronic conditions
Why Research Is Critical in Rare Diseases
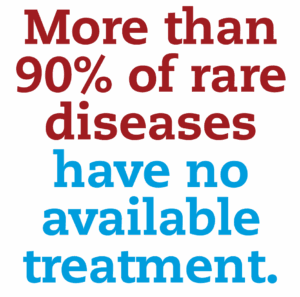
Because rare diseases affect fewer people, it can be challenging to find enough volunteers for studies. This includes conditions such as Tardive Dyskinesia (TD), where smaller populations make participation even more valuable. With many rare conditions lacking approved treatments, even a small number of people choosing to take part can advance discoveries that change the outlook for an entire community.
Why Diversity in Research Matters
Diversity in clinical research ensures treatments reflect the people who will use them. People of different ages, races, genders, cultures, and lived experiences may respond differently to care. Inclusive participation helps uncover disparities while building trust by showing that everyone’s needs and experiences matter.
The Journey from Idea to Treatment
Every treatment available today began as an idea that had to be tested with care, intention, and the partnership of people. Researchers move through several stages of clinical trials to understand how a treatment works and whether it can truly improve lives. Because only a small number of medications make it all the way to approval, every study and every participant plays a meaningful role in shaping the future of care.
Phase I
Understanding Safety
Small group of volunteers. Focus on safety, dosage, and how the medication behaves in the body.
Phase II
Learning How It Helps
A larger group with the condition. Measures effectiveness and identifies side effects.
Phase III
Strengthening the Evidence
Hundreds or thousands of participants. Confirms effectiveness, monitors side effects, and compares to existing treatments.
Phase IV
Tracking Real-Life Impact
Conducted after approval. Follows people using the medication in everyday settings.
For a more in-depth overview, you can review the PDF version of this process.
Should I Take Part in Clinical Research?
Joining a study is a personal decision. People participate for many reasons, including the hope for better options, access to new treatments, and a desire to help others.
- Participation is always voluntary
- You can leave a study at any time
- You will continue to receive health care whether you participate or not
- The research team will explain all potential risks and benefits
Questions to Ask Before Joining a Clinical Trial
Before deciding to join a clinical trial, take time to understand the study and what participation means for you. You deserve clear information, and it’s always okay to ask questions at any stage.
Understanding the Study
- What treatment is the clinical trial studying?
- Who is paying for the trial?
- Has this been approved by the Institutional Review Board (IRB)?
- How does the study make sure the process is transparent and culturally responsive for participants?
Location, Time Commitment, and Logistics
- How long will the trial last?
- Where will the trial take place? Is hospitalization required?
- How much time will I have to commit to participating in the clinical trial?
- How often will the study personnel need to see me? How long will the visits be?
- What time of day should I schedule appointments?
- Can I continue to see my own doctor while in the trial?
Treatment Plan and Medication Questions
- Will I have to discontinue my present treatment(s)?
- If so, could this harm my health?
- If I can stay on my present medication(s), will taking experimental medication be bad for my health?
- If I am involved in a “crossover” clinical trial, can I go back to the first treatment I received if it worked better than the second treatment?
- Will everyone involved in the clinical trial get the same treatment?
- If not, what are my chances of receiving an existing treatment or a placebo?
- What else will I have a chance of receiving?
- If I don’t receive the new treatment during the clinical trial, will I have an opportunity to try it at the end?
- If I receive the new treatment and it helps me, will I be able to continue using it after the trial ends?
- If I have bipolar disorder, what will happen if the treatment makes me rapid cycle or manic?
Risks, Safety, and Emergency Protocols
- What are the possible side effects and/or risks?
- What will be done if I experience side effects during the clinical trial?
- Will I be promptly informed of any findings that might make me want to drop out—for example, if the experimental medication can make me ill?
Confidentiality, Rights, and Legal Protections
- What procedures are in place to ensure my confidentiality?
- Can I drop out at any time, for any reason?
- Can I sue if I suffer injury?
Costs, Compensation, and Support
- Are there any fees associated with the study?
- Will I be paid for my participation?
- What kind of follow-up care will I receive after the trial is completed?
Where to Find Out About Clinical Trials
Depression and Bipolar Support Alliance does not endorse or recommend any particular clinical trial. Patients should discuss all options with their health care providers and family members before beginning any trial.
Ads for clinical trials appear in newspapers, magazines, on billboards, on the radio, and on television. These ads usually do not adequately describe the trials, but they usually give you contact information. Be sure to review possible risks and benefits with your doctor, family, and friends before participating in any trial.




